The nucleic acid synthesis is carried out using the solid phase sialic amide triglyceride method, whereby the 3′ end of the DNA is immobilised on a solid phase substrate and nucleotides are added in the 3′ to 5′ direction until the desired DNA fragment is synthesised. This differs from DNA synthesis by the application of DNA polymerase.
The 3′ end of the first base is immobilised on CPG during synthesis, the 5′-OH of the next base is protected with di-p-tolyl trityl DMT, the amino group on the base is protected with benzoic acid and the 3′-OH is then activated with an amino phosphate compound. 1 base of the 5 5′-OH of 1 base and 3′-OH of the next base form a phosphite triglyceride, which is then oxidised with iodine to a phosphate triglyceride, the protectant on the second base 5′-OH is removed by adding dichloroacetic acid DM cycles through the addition of the next base, and after synthesis the protectant on the 5′-OH is removed with a weak acid The fragment is disconnected from the solid resin with concentrated ammonium hydroxide, the protectant is removed from the base with concentrated ammonium hydroxide under heating, the ammonium hydroxide is removed, the fragment is vacuum dried, and the nucleic acid is purified by liquid chromatography or PAGE.
Steps of Oligo Synthesis
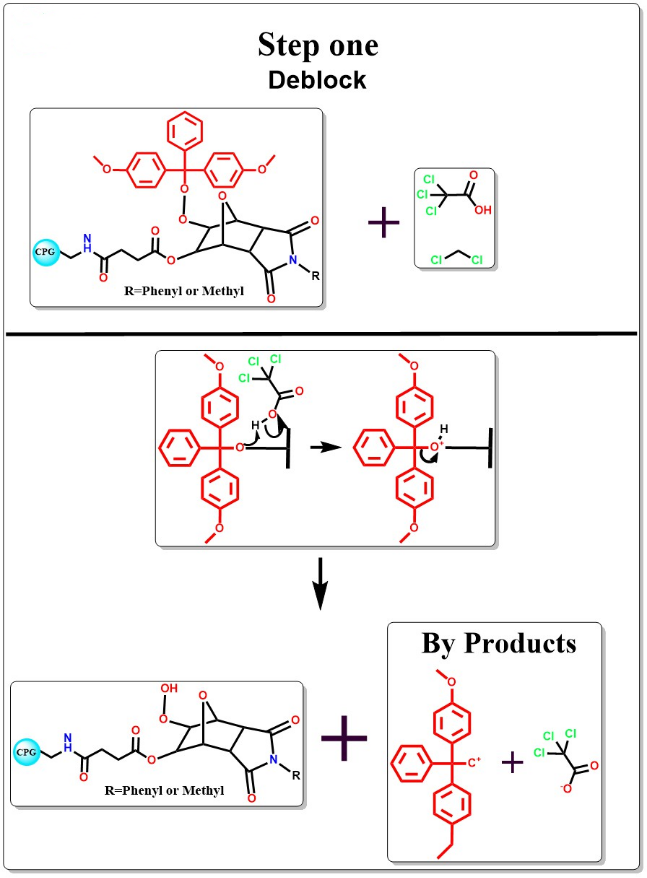
Deblocking
The 5 end DMT protecting group is removed by adding TCA solution.
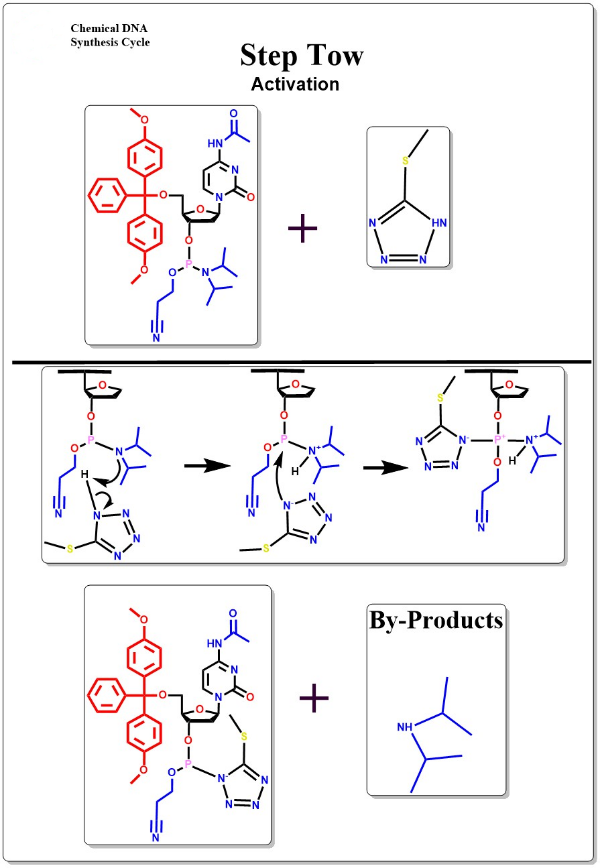
Activation
Mixing the activator with the oligonucleotide monomer to form the active oligonucleotide monomer intermediate.
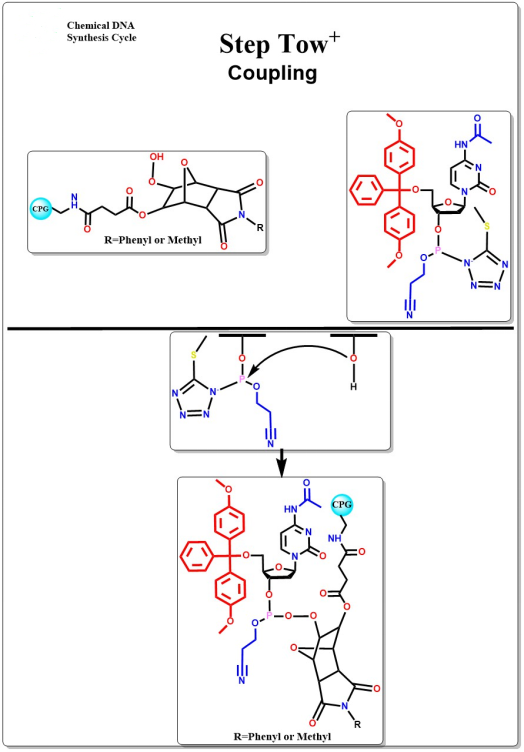
Coupling
The 5-terminal hydroxyl group reacts with the active oligonucleotide intermediate in an anti-generative condensation reaction to form an unstable phosphite triglyceride bond.
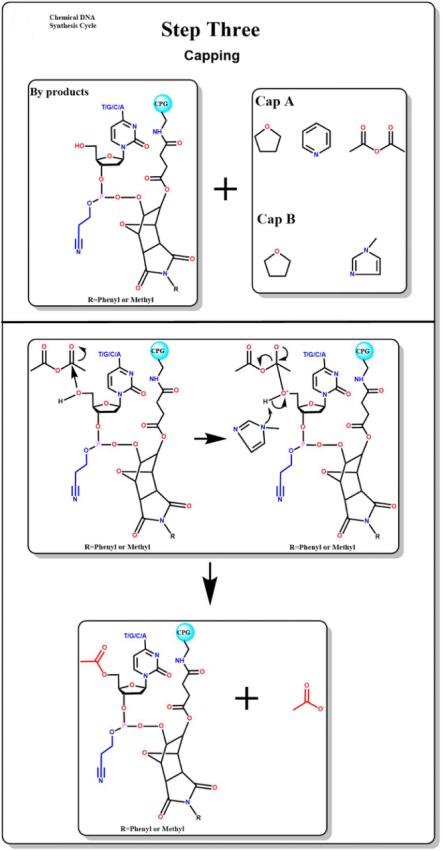
Capping
The addition of a capping agent performs an acetylation capping reaction with excess hydroxyl groups that are not involved in the condensation reaction.
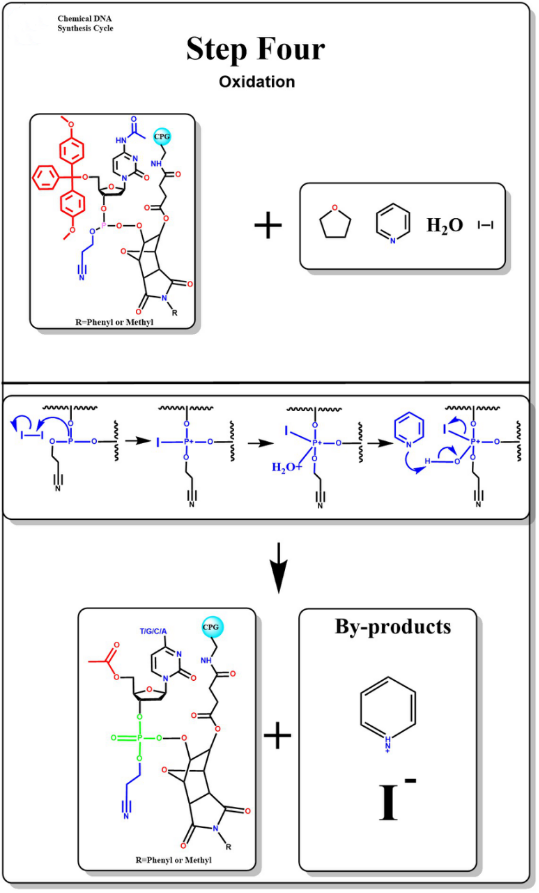
Oxidation
The addition of iodine solution containing water reacts with unstable phosphite bonds by oxidation to form stable phosphoric acid triglyceride bonds.
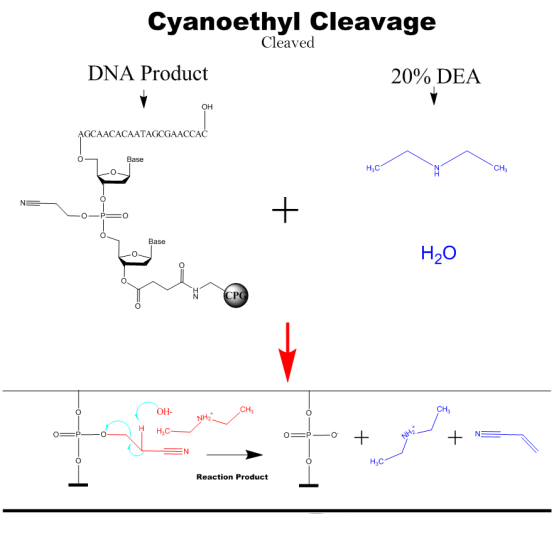
Cyanoethyl Cleavage
The nucleic acid product after synthesis, with the addition of DEA solution to remove the cyanoethyl group from the phosphate triglyceride bond.
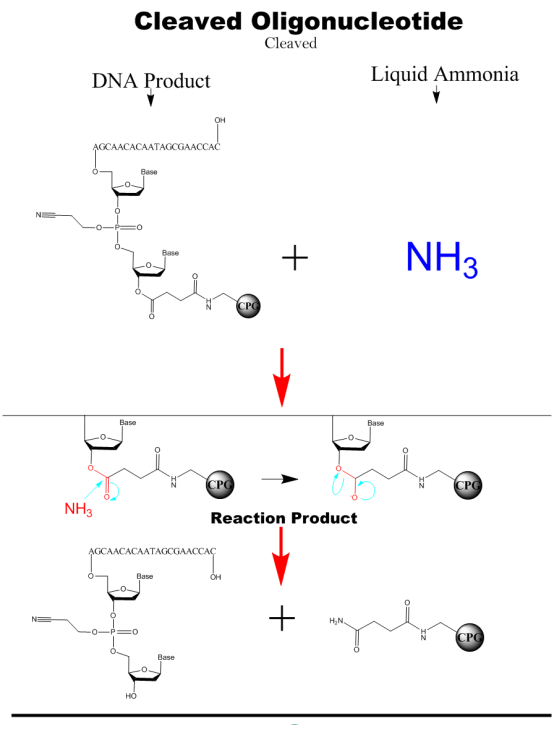
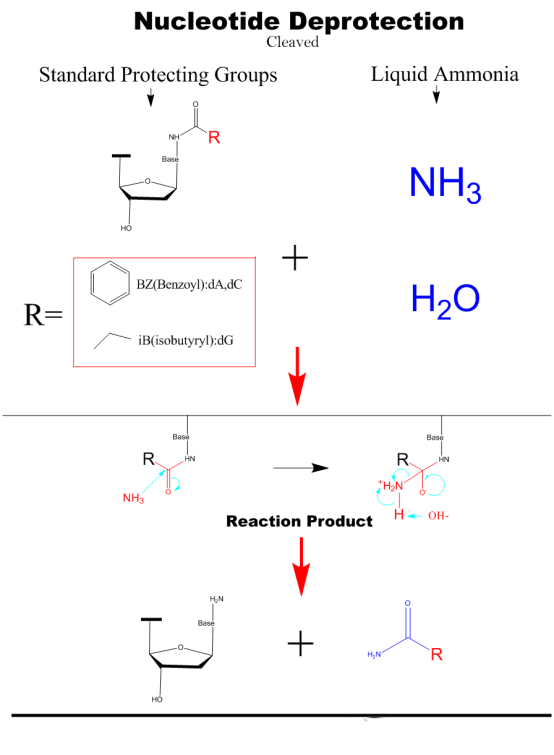
Cleaved&Deprotection
The nucleic acid fragments are cleaved from the solid phase carrier by adding a concentrated ammonium hydroxide solution and then heated to remove the protecting groups from the hydrogen bonds of the bases.
Post time: Nov-21-2022